The broader picture
Every day nutritionists face the need to balance the food energy intake with the metabolic needs of the patient.
In the still predominant practice of low-calorie diets, for example, the aim is to reduce more or less significantly the daily energy intake provided by food (typically measured in Kcal) compared to the estimated expenditure based on parameters such as age, sex, weight and activity of the patient.
Although it is impossible for nutritionists to precisely assess the body energy balance, there is unfortunately a general oversimplification of the subject, almost as if to mean that, since accurate estimates are not available, it is worth neglecting the issue and acting in a rough way.
The mistake in the hypothesis
Various equations and theories try to approximate the daily energy requirements based on anthropometric parameters, age, sex or type of physical activity. However, these tools have clear intrinsic limitations that mostly lead to an overestimation of the patients’ metabolism, even in terms of basal metabolic rate.
It should be noted here that basal metabolic rate (or BMR, BIA-ACC) means the amount of energy (usually expressed in daily Kcal) consumed by an individual in conditions of maximum physical and mental rest, in an environment with a comfortable temperature and fasting for about twelve hours. This being said, it is clear that an energy supply equal to at least the BMR is the minimum requirement to avoid an energy deficit in the body.
Clearly, too, the daily energy demand is necessarily higher than the BMR, which is the minimum energy that is required for the survival of the body’s cellular mass, provided that rest is absolutely ideal.
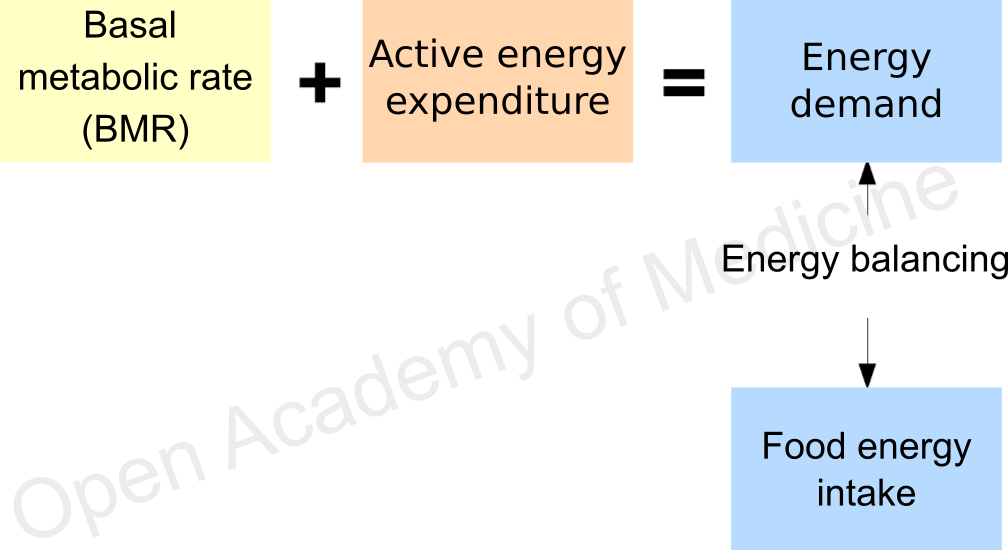
Figure 1: balance between energy intake and demand
In summary, the picture resulting from the above is shown in Figure 1: considering the sum of BMR and active energy expenditure, the balance of daily energy intake/expenditure should be simple enough. Not that this is wrong in absolute terms, on the contrary: the issue is defined correctly, the problem rather lies in the practice – a practice that, sometimes out of necessity, sometimes due to misplaced confidence, relies too often on standard equations, overlooking the inaccuracy of these tools.
Let’s put aside for a moment the issue of active energy expenditure, although not simple, and let’s focus only on the assessment of the basal metabolic rate. The criticism that we want to bring forward is, in the end, the following: are two patients of the same age, of the same sex, and anthropometrically similar, certainly characterized by an equal or similar BMR? The short answer is: “no, not at all”.
Metabolic changes
Normally, the patient is not “healthy as a horse”. Although it may sound a bit ironic, this concept should emphasize how unusual it would be for a perfectly healthy person to come to a nutritionist’s practice to get advice on how to eat.
Obesity, diabetes, functional gastroenteric disorders are only some of the most common examples of conditions for which people request some kind of help in this area, and they all are characterized by more or less significant changes in the physiological balance of the endocrine system and of body composition, as well as by the onset of various symptoms or by the chronicity of inflammatory processes.
Events that are attributable to this sphere occur very easily even in the absence of symptoms: the scientific literature has long since clarified, for example, that the increase and persistence of stressors over time (be they physical, psychosocial, exogenous or endogenous) lead, in the long run, to changes in the circadian rhythmicity of stress hormones (glucocorticoids, cortisol - HPA axis index - BIA-ACC); at the onset of this imbalance, an apparently healthy subject would therefore experience, to some extent, a lower ability to metabolize carbohydrates due to cortisol-insulin antagonism, for example. Pushing the hypothesis forward, this would lead to a change in the body composition to the detriment of the lean mass (FFM, Fat Free Mass, roughly made up of muscular, bone tissue and internal organs excluding fat - BIA-ACC), which is one of the main factors that define the BMR.
A different issue, but leading to parallel outcomes, pertains to the alteration of extracellular pH, such as the tendency to acidosis that is related to the presence of chronic inflammatory processes: as is well known, acidosis involves the progressive decay of the enzymatic activity and hence of the organic absorption of nutrients (for example caused by food with a positive PRAL). In these conditions, what is the point of taking nutrients that are not balanced with the real metabolic capacities? It would rather be advisable to stimulate the metabolism, so as to avoid a decrease in nutrient absorption leading to the loss of FFM.
There are a very large number of interactions, in addition to those briefly mentioned, that can cause changes in metabolism, only a few of which are shown in Figure 2.
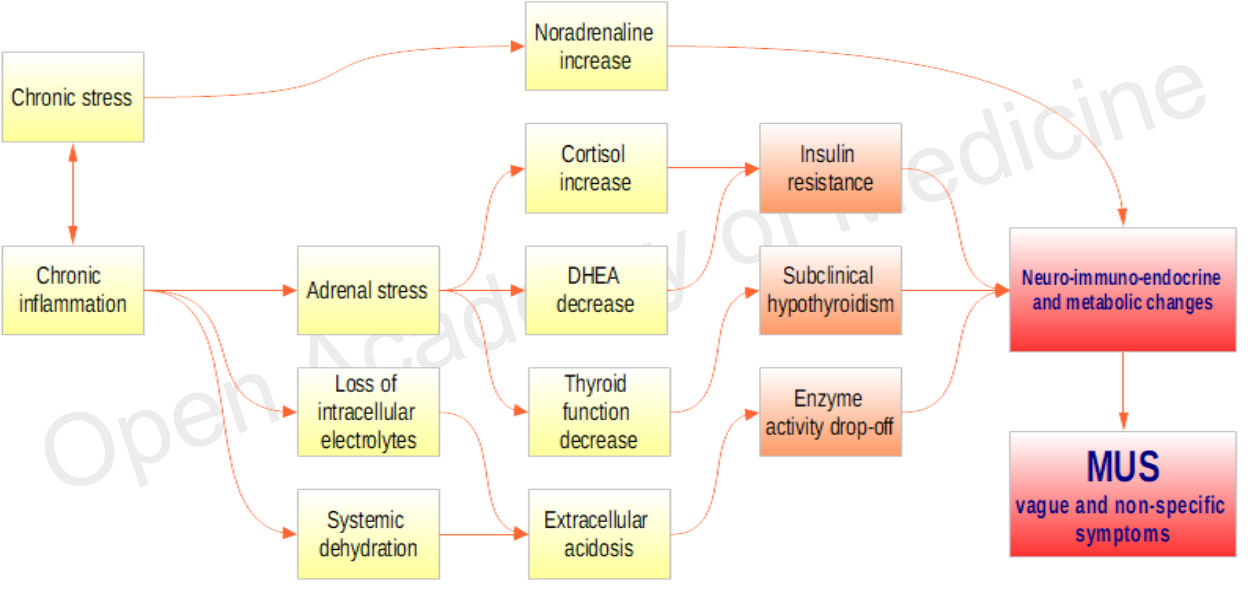
Figure 2: Examples of interactions involved in metabolic changes
In many cases, the onset of these events triggers so-called “vague and non-specific” symptoms (or MUS, Medically Unexplained Symptoms, see Table 1 - MUS [46]), which are often the first warning signal for the patient.
It should be noted, however, that the identification of symptoms relies on the patient’s subjective perception. Hence, even in the absence of disorders it should not be taken for granted that statistical equations are applicable to any patient.
Table 1: Vague and Non-specific Symptoms (MUS) – simplified, from Vague and Non-specific Symptoms Self-Assessment Form – MUS® [46].
Towards an improvement
The availability of detailed and exhaustive information on the patient’s status is by no means a given, and it would be in any case an uneconomical and lengthy option requiring a long series of laboratory tests. This is not, in most cases, a situation that you typically come across in a clinical nutrition practice.
However, it is possible to obtain objective parameters that go beyond a mere anthropometric survey and allow to make decisions more effectively. A rapid non-invasive analysis of body composition, if performed with an adequate degree of precision, can say a lot about a patient’s health.
The parameters that can be identified through the clinical analysis of body composition (using the BIA-ACC tools in combination with the PPG Stress Flow device for the analysis of the autonomic nervous system) provide important measures of the patient’s hydration level, his adipose tissue content, his metabolic capacity, his degree of chronic systemic inflammation.
The BIA-ACC test, as now demonstrated by scientific literature, offers a good degree of accuracy in estimating body composition and BMR, and is a feasible solution in almost any situation, thanks to its cost-effectiveness and ease of management. Having a more accurate and correct measurement is undoubtedly the first step towards improving the results obtained in terms of nutritional balance, reducing the risk of unbalancing the calorie intake compared to the real needs and the real metabolic capacities of the patient.
Currently, the technology applied to clinical nutrition has reached more advanced stages; an example in this sense is the integrated platform BioTekna Plus (“Clinical Nutrition” application), which has been available for some time and is accessible to a wide community of users. This platform allows to perform nutritional analyses in real time starting from BIA-ACC, PPG Stress Flow parameters as well as from interviews on the patient’s vague and non-specific symptoms (MUS) and eating habits (Eating Habits).
Among the data collected by the BioTekna Plus integrated platform, the analysis of MUS offers a lot of information about the patient, because it allows to further characterize the data obtained from the BIA-ACC and PPG Stress Flow examination, up to the possibility of outlining a profile of circadian hormonal rhythmicity (see HPA axis index - BIA-ACC and SNS, ANS - PPG Stress Flow).
In practical terms: how useful can it be to know when a certain type of food is well tolerated by the patient throughout the day? How useful can it be to promote, through precise nutritional choices, phases of pancreatic rest, or thyroid activity? The potential improvements obtained under nutritional therapy by such capacities are of inestimable value. They can make a difference between a general weight loss by an obese patient and a selective loss of fat mass, or between the need to use hypoglycemic drugs at every meal and the possibility of minimizing hyperglycemic peaks.
The Biotekna Plus integrated platform (Figure 3) fills the gap between instrumental data and the nutritionist’s strategies. These can be adapted to the characteristics of the individual patient, thanks to the ability to calculate the trend of the metabolic response in 24 hours (glycemic load, Food Insulin Index, AGEs, PRAL) both in the investigation phase – i.e. checking the patient’s typical metabolic behavior and in relationship with his habits – and in the therapeutic hypothesis phase, thus allowing the nutritionist to verify the appropriateness of his choices.
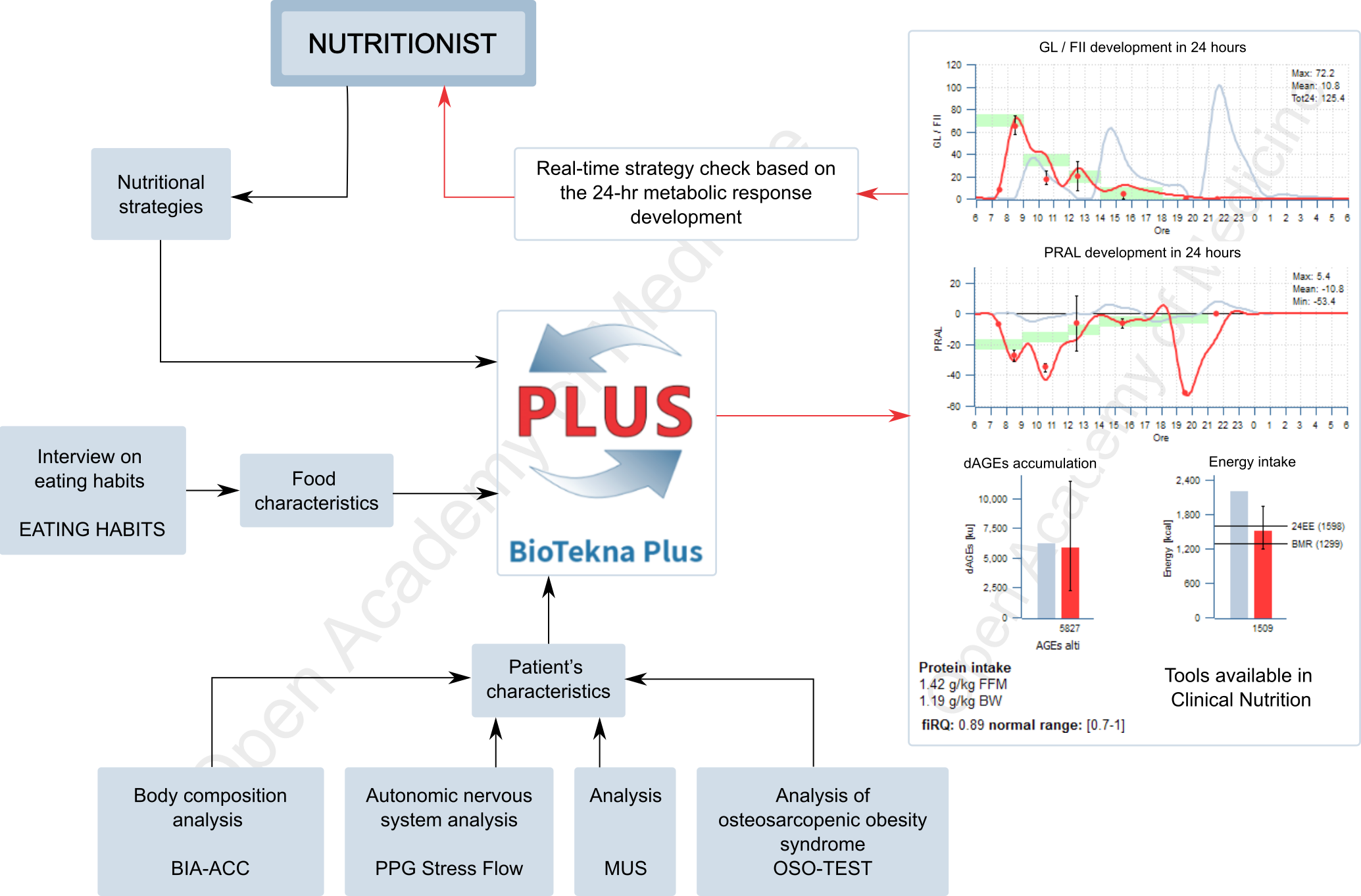
Figure 3: Clinical nutrition application integrated in the BioTekna Plus platform.
The use of the integrated platform BioTekna Plus facilitates the nutritionist’s task because, in addition to simplifying the balance of the daily energy intake based on a correct BMR estimate, it helps evaluate which are the most appropriate moments (nutritional sequencing) for food intake, in order to stimulate the metabolism in the most favorable phases and support the recovery of the physiological hormonal rhythmicity – this aspect being fundamental for the recovery of the well-being of patients characterized by a very low basal metabolic rate, for whom a too strong calorie reduction could even be harmful.
Authors: Dario Boschiero - Date: 04/12/2020
Attention: these contents can be freely used for personal learning purposes only. The use is regulated by Law No. 633/1941 and subsequent amendments, as well as by the copyright and patent legislation in force. Any use for commercial and profit-making purposes is forbidden.
References